Rules for Classification of Medical Devices
Rules for Classification of Medical Devices
(Decree No.15 of China Food and Drug Administration)
Issued on July 14, 2015
Decree No.15 of China Food and Drug Administration
The Rules for Classification of Medical Devices, adopted at the executive meeting of China Food and Drug Administration on June 3, 2015, is hereby promulgated and shall be effective as of January 1, 2016.
Minister: Bi Jingquan
July 14, 2015
Rules for Classification of Medical Devices
Article 1 The Rules is stipulated in accordance with the Regulations on Supervision and Administration of Medical Devices to standardize the classification of medical devices.
Article 2 The Rules aims at directing the formulation of classification catalogue of medical devices as well as determining the classes for new medical devices.
Article 3 The terms used in the Rules are defined as follows:
(1) Intended purposes
Effects that shall be achieved by using a medical device in accordance with instructions, labels or publicity materials.
(2) Non-active medical device
A medical device of which the effects are achieved not relying on electric or other forms of energy, but may use the energy directly generated by human body or gravity.
(3) Active medical device
Any medical device that operates on electric or other forms of energy excluding the energy directly generated by human body or gravity.
(4) Invasive device
A medical device which is wholly or partially inserted into human body through body surface by way of surgery, and contacts such parts as tissue, blood circulation system and central nervous system. It may include an interventional operation instrument, a disposable sterile surgical device and a device which may remain in human body temporarily or for a short term, ect.. The invasive device in the Rules does not include a reusable surgical instrument.
(5) Reusable surgical instrument
A non-active medical device intended for surgical use by cutting, drilling, sawing, scratching, scraping, clamping, retracting, clipping, without connection to any active medical device and which can be reused after appropriate procedures have been carried out.
(6) Implantable device
A medical device that is wholly or partially inserted into the human body, the orifices or stoma by way of surgery, or used as a substitute for the epithelial surface or ocular surface, which may remain in the body for at least 30 days after the surgery or may be absorbed by human body.
(7) Body-contacting device
A medical device that directly or indirectly contacts or can be inserted into the body of the patient.
(8) Service life
1. Continuous service period: The uninterrupted actual action period of medical device according to intended purpose.
2. Temporarily: The intended continuous service period of medical device is less than 24h.
3. Short-term: The intended continuous service period of medical device is at least 24h but less than 30 days.
4. Long-term: The intended continuous service period of medical device is at least 30 days.
(9) Skin
Unwounded skin surface.
(10) Orifices (stoma)
Natural orifices and permanently artificial openings of human body such as oral cavity, nasal cavity, esophagus, external auditory canal, rectum, vagina and urethra.
(11) Trauma
Compromised structural integrity of tissue or dysfunction of organism caused by various factors.
(12) Tissue
In vivo tissue, including bone, dental pulp or dentin, but not including blood circulation system and central nervous system.
(13) Blood circulation system
Blood vessels (except the blood capillary) and the heart.
(14) Central nervous system
Brain and medulla spinalis.
(15) Stand alone software
A software with one or more medical purposes which may complete the intended purposes without using a medical device hardware and runs on the universal computing platform.
(16) Medical device with measuring functions
A medical device intended to measure the physiological, pathological and anatomic parameters or make quantitative determination on the energies/substances entering or leaving the human body. The measuring results shall be accurately quantified, and the accuracy of such results may have obvious effects on patient's health and safety.
(17) Chronic wound
A wound which is not healed for a long time due to various reasons, such as venous ulcer, arterial ulcer, diabetic ulcer, traumatic ulcer and pressure ulcer, etc..
Article 4 According to degree of risk (from low to high), the classifications of medical devices are divided into class I, class II and class III in due order.
The risk degree of a medical device shall be determined comprehensively according to the intended purpose, structural characteristics, pattern of use, status of use as well as whether the device is body contacting.
Article 5 According to the factors which may influence the degree of risk of medical devices, medical devices are divided as follows:
(1) Non-active medical devices and active medical devices, according to structural characteristics.
(2) Body-contacting device and non-body-contacting device, according to whether they are in contact with human body.
(3) Patterns of use of medical devices, according to their different structural characteristics and whether are in contact with human body, include:
Non-active body-contacting devices: liquid transportation devices, blood and other body fluids alternation devices, medical dressing, invasive devices, reusable surgical devices, implantable devices, contraceptive and family planning devices and other non-active body-contacting devices.
Non-active non-body-contacting devices: nursing devices, devices for medical device disinfection and cleaning, and other non-active non-body-contacting devices.
Active body-contacting devices: energy treatment devices, diagnostic and monitoring devices, liquid transportation devices, ionized radiation devices, implantable devices, and other active body-contacting devices.
Active non-body-contacting devices: clinical laboratory instruments, stand alone software, instruments for medical device disinfection and sterilization, and other active non-body-contacting devices.
(4) The status of use or the effects generated of medical devices, according to their different structural characteristics, whether are in contact with human body and patterns of use, include:
Non-active body-contacting devices: temporarily used, the device used for short term and the device used for long term, according to service life. The positions in contact with human body include skin or orifice (stoma), trauma or tissue, blood circulation system or central nervous system.
Non-active non-body-contacting devices: little impact, minor impact, and significant impact, according to the impact on treatment.
Active body-contacting devices: minor, moderate and serious injuries, according to the degree of injuries caused by malfunction of these devices.
Active non-body-contacting devices: little impact, minor impact, and significant impact according to the impact on treatment.
Article 6 The classification of medical devices shall be conducted in accordance with the Table for Determination of Medical Device Classification (see Annex). Where the following situations exist, the classification shall be made in combination with the following principles:
(1) Where two or more classes are applicable to one medical device, the one representing highest risk degree shall be adopted; the class of a medical device kit composed of multiple medical devices shall stay with the one representing highest risk degree in the kit.
(2) For a medical device which may be used as an accessory, its classification shall be made with comprehensive consideration of the impact of the accessory to the safety and effectiveness of the major medical device used with. If the accessory has significant impact on the major medical device used with, the class of such an accessory shall not be lower than the major medical device used with.
(3) A medical device used to monitor or influence the essential functions of another medical device, its class shall be identical to the medical device being monitored or influenced.
(4) A drug-device combination product with its major effects is as a medical device, it shall be regarded as a class III medical device.
(5) An absorbable medical device shall be regarded as a class III medical device.
(6) An active body-contacting device with significant impact on medical results shall be regarded as a class III medical device.
(7) In any of the following cases, medical dressings shall be regarded as a class III medical device: the medical dressing is intended to have a function to prevent tissue/organ adhesion; is used as artificial skin; is to contact with the deep dermis or the injured trauma of tissue below dermis; is used to heal chronic wound, or may be wholly/partially absorbed by human body.
(8) A medical device supplied in sterile state, its class shall not be lower than class II.
(9) An orthopedic medical device which is used to actively apply a sustained action force to human body in such action modes as pulling, strutting, twisting, holding and bending and may dynamically adjust the fixed position of limb (not including a medical device with fixation and supporting function only, a medical device used in conjunction for temporary orthopedics in the surgery or a medical device used for limbs orthopedics after surgery or in other treatment), its class shall not be lower than class II.
(10) A medical device with measuring function, its class shall not be lower than class II.
(11) A medical device intended for the treatment of a certain disease, its class shall not be lower than class II.
(12) A reusable surgical device used in surgical operations under endoscope, such as tissue picking up, cutting or stone removing, etc., shall be regarded as a class II medical device.
Article 7 In-vitro diagnostic reagents shall be classified in accordance with corresponding regulations.
Article 8 The China Food and Drug Administration shall analyze and evaluate the risk changes timely according to the situation in medical device production, distribution and use, and adjust the medical device classification catalogue.
Article 9 The China Food and Drug Administration may organize a medical device classification experts committee to formulate and adjust the medical device classification catalogue.
Article 10 The Rules shall be effective as of January 1, 2016. The Provisions for Medical Device Classification promulgated on April 5, 2000 (Decree No. 15 of the former State Drug Administration) is annulled simultaneously.
Annex: Table for Determination of Medical Device Classification
Annex
Table for Determination of Medical Device Classification
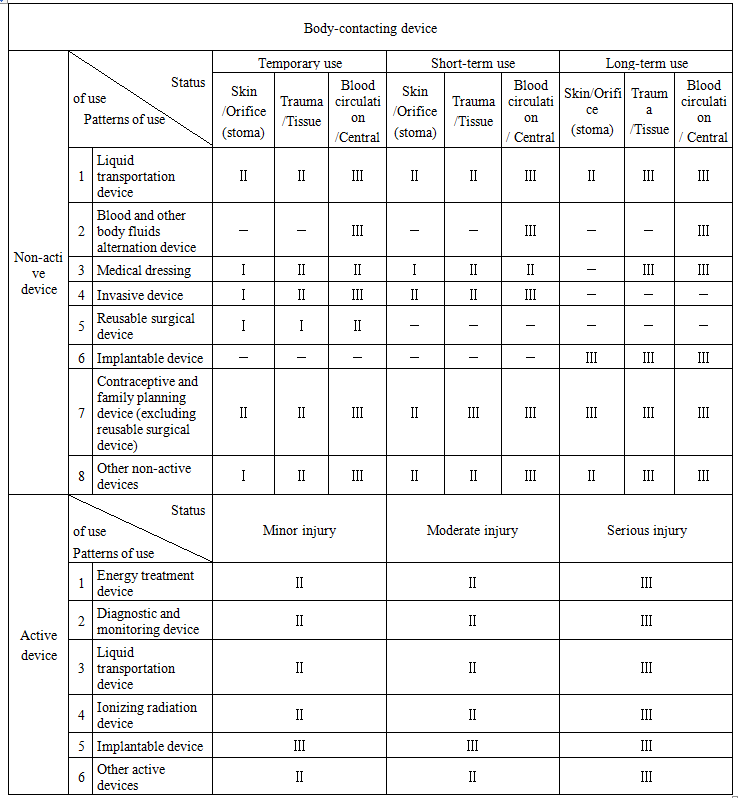
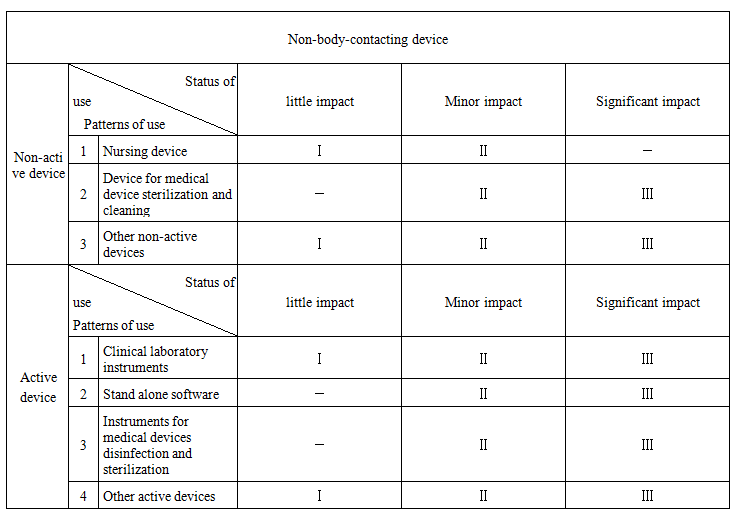
Notes: 1. “I”,“II”and“III”herein respectively refer to class I, II and III medical devices.
2. “-”herein means the situation is inapplicable to any class of medical devices.
Note: In case of any difference in interpretation between the English version and the Chinese version, the Chinese version shall prevail.



