The 2018 Drug Review Annual Report released
( V ) Completion of administrative examination and approval tasks
In 2018, CDE completed a total of 5,860 administrative examination and approval tasks, covering 1,808 direct administrative examination and approval tasks (i.e., supplementary applications dispense with technical review) with an average time limit for examination and approval of 12.3 working days, far less than the statutory 20- day time limit for administrative examination and approval, 1,656 of which were completed within the statutory 20-day time limit, and the average completion rate within time limit was 92% in 2018. CDE completed the administrative examination and approval for 4,052 tasks with technical review (viz., IND, import re-registration and supplementary applications requiring technical review, etc.), with an average time limit of 18.6 working days, which also curtails the statutory 20- day time limit, the average completion rate within time limit was 84% in 2018. (Note:
The above-mentioned 4,052 tasks exclude those that have been accepted and reviewed prior to the joint review & approval of APIs, excipients and packaging materials and transferred to registration platform management of APIs, exipients and packaging materials.
(VI) Incorporation of varieties for prioritized review
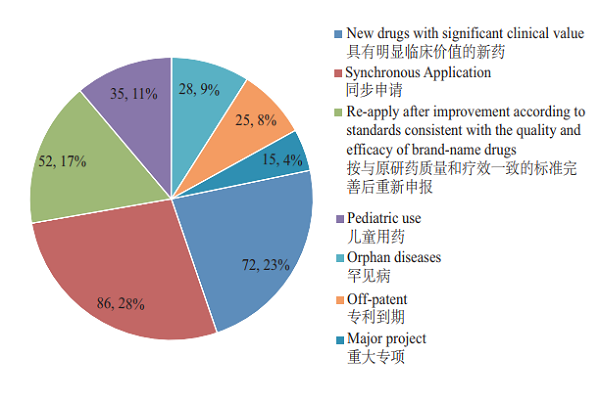
As per the Opinions on Fueling Pharmaceutical Innovation via Prioritized Review & Approval (SYJYHG [2017] No. 126) of the former China Food and Drug Administration (hereinafter referred to as the former CFDA), in 2018, CDE will have incorporated a total of 313 registration applications into prioritized review process, covering 63 applications for pediatric use and orphan diseases. Of the registration applications that were included in the prioritized review in 2018, the proportion of Synchronous Applications (which refers to 5th case in the Scope of Prioritized Review & Approval (A) (CFDA No. 19), viz., drugs whose clinical trials have been simultaneously applied and approved in the European Union and the United States, or drugs that are manufactured in China with the same production line, and simultaneously applied for marketing in EU or US and have passed the on-site inspection of their drug evaluation and approval authorities) was the largest, accounting for 28%, followed by new drugs with significant clinical value, accounting for 23%. The registration applications incorporated into prioritized review process are detailed in Figure 24.
Figure 24 Registration applications incorporated into in prioritized review process
2. Completed review of varieties subject to prioritized process In 2018, a total of 83 varieties (in generic terms) were expedited for marketing approval via prioritized review process, such as Albuvirtide for Injection, Danoprevir Sodium Oral Tablets for hepatitis C treatment, and small-molecule angiogenesis inhibitor
Fruquintinib Capsules for the treatment of advanced colorectal carcinoma, and other innovative drugs independently researched and developed in China, the list of specific varieties is shown in Annex 3.
(VII) Communication and exchanges (omitted)
III . Encouraging innovation and safeguarding medication safety for the public (omitted)
IV. Programs and progresses (omitted)
V. Key objectives for 2019 (omitted)



