The 2018 Drug Review Annual Report released
(IV) Completion of the review of registration application for biologicals
1. Overall situation
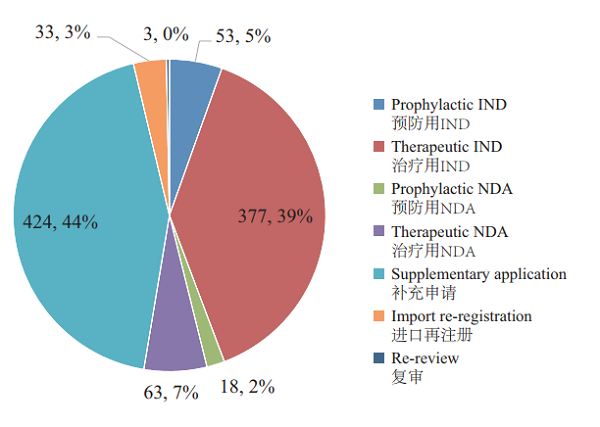
A total of 971 applications for biologics registration were completed by CDE, covering 53 IND applications for prophylactic biological products (prophylactic IND), 377 IND applications for therapeutic biological products (therapeutic IND), 18 NDAs for prophylactic biological products (prophylactic NDA), and 63 NDAs for therapeutic biological products (therapeutic NDA).
See Figure 21 for details of the various registration applications for biologicals that have been reviewed.
Figure 21 CDE-completed reviews of various registration applications for biologicals in 2018
2. Approved reviews
CDE reviewed and approved 33 prophylactic INDs, 316 therapeutic INDs; 11 prophylactic NDAs and 30 therapeutic NDAs. The details of the various registration applications for biological products completed in 2018 are shown in Table 3. The IND and NDA approval of biological products are compared with the previous three years (in terms of acceptance number) as shown in Figure 22.
Table 3 Details of completed reviews for various types of registration applications for biological products in 2018
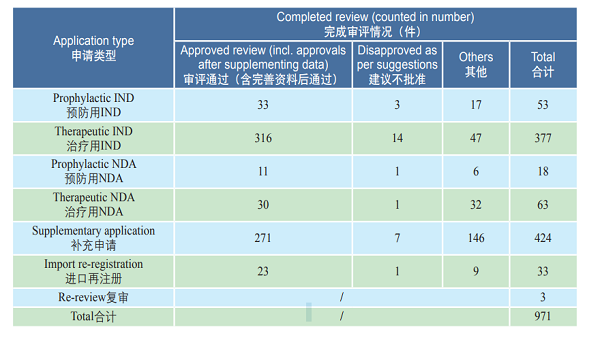
Figure 22 Comparison of the number (counted by acceptance numbers) of IND and NDA review approvals for biologicals in 2015-2018
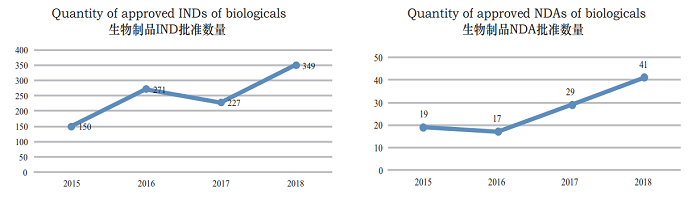
CDE reviewed and approved 349 biological INDs, for details of the distribution of therapeutic areas of approved therapeutic biological INDs, see Figure 23 (omitted).



