The 2018 Drug Review Annual Report released
(II) Completion of the review of chemicals registration applications
1. Overall situation
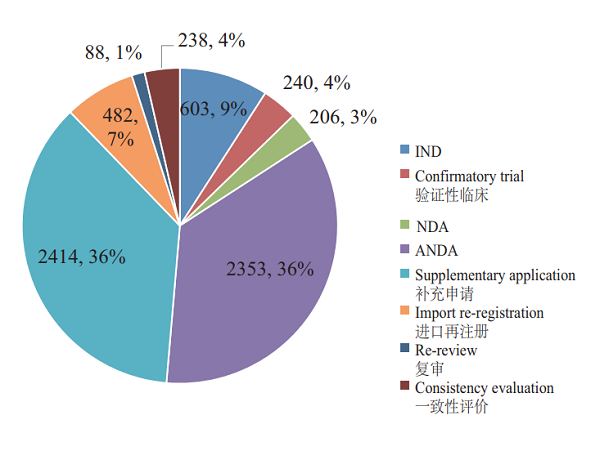
CDE completed the review of registration applications for 6,624 chemical drugs, including 843 clinical applications (IND and confirmatory trial), 206 NDAs, and 2,353 ANDAs. See Figure 14 for details of the various registration applications for chemical drugs that have been reviewed.
Figure 14 Reviewed registration applications for various chemical drugs in 2018
2. Approved reviews
CDE completed 206 reviews of chemicals NDA, 132 (in terms of acceptance number) of which were approved, a comparison with the previous three years is shown in Figure 15. For details of various registration applications for chemical drugs with completed reviews in 2018, see Table 1.
Figure 15 Comparison of the number of approved reviews of chemicals NDA in 2015- 2018
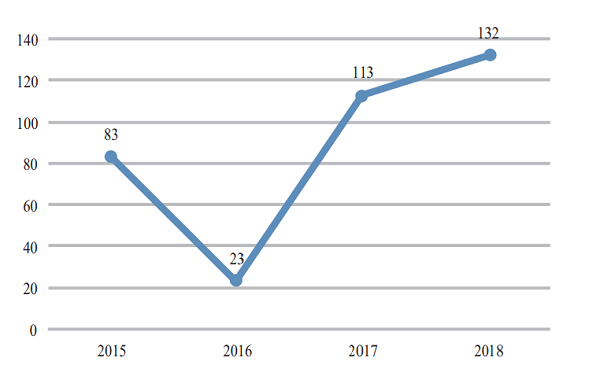
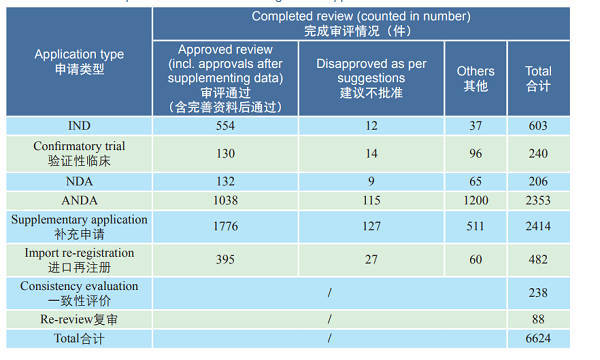
Table 1 Details of completed reviews for various registration applications of chemicals in 2018
CDE completed reviews for 603 applications for chemicals IND, 554 of which were approved, including 449 IND applications for Class 1 innovative drugs (involving 172 varieties). The numbers of IND approved for Class 1 chemical innovative drugs in 2015-2018 (in terms of variety) are shown in Figure 16.
CDE reviewed and approved the INDs of 172 varieties of innovative drugs, mostly for antitumor, digestive system, endocrine system and anti-infective use, accounting for 68% of the total. The distribution of indications for innovative chemicals (in terms of variety) with approved INDs is shown in Figure 17.
Figure 16 The number of IND applications approved for chemical innovative drugs in 2015- 2018 (in terms of variety)
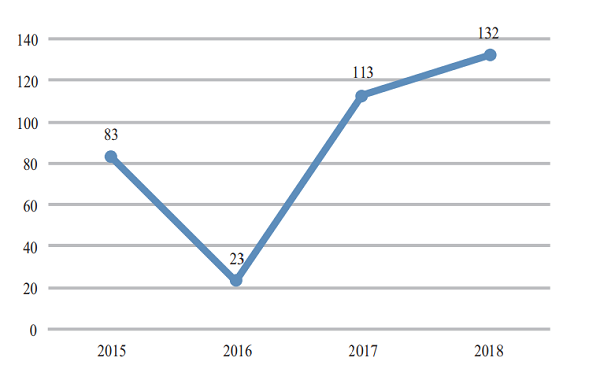
Figure 17 Distribution of indications for innovative chemicals (in terms of variety) with INDs approved in 2018
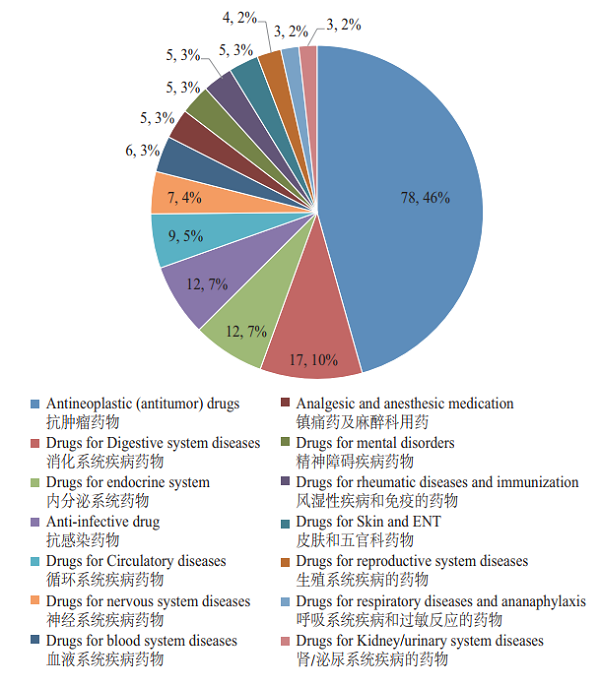
Note: Some of the innovative chemicals involve multiple indications and are classified into different indication groups, so therefore the sum of each indication group of INDs is more than 172 in the picture above.



