The 2018 Drug Review Annual Report released
II. Review & approval of drug registration applications
(I) Overview of accomplished review & approval
1. Review & approvals accomplished in 2018 As of the end of 2018, CDE had accomplished over 90% of the review & approval of registration applications for TCM, chemicals and biologicals, thus basically completed the objective set forth in Doc. No. 44 requiring the completion of review & approval in 2018 within the prescribed time limit. Of the grand sum of 9,796 registration applications reviewed and approved in 2018, 7,988 were subject to technical review (incl. 4,052 administrative approval tasks requiring technical review), and 1,808 to direct administrative approval.
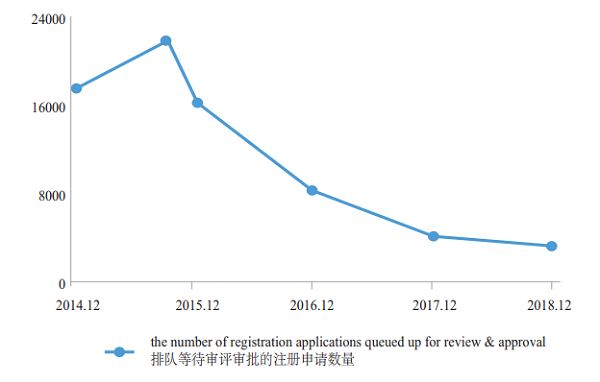
The number of pending registration applications has dropped from nearly 22,000 at the peak of September 2015 to 3,440 (excluding those applications whose review have been completed and are pending for the Applicants' supplementary dossiers) by the end of 2018, further consolidating the accomplishment of liquidating the backlog of registration applications as required by Doc. No. 44. The changes in the number of registration applications queued up for review & approval in 2014-2018 are shown in Figure 11.
Figure 11 Changes in the number of registration applications queued up for review & approval in 2014-2018
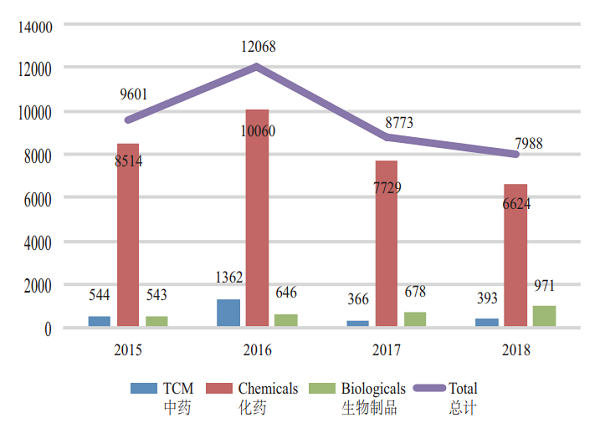
Of the applications with completed review, 6,624 were registered for chemical drugs, accounting for about 83%. The completion status of various types of drug registration applications in 2015-2018 is detailed in Figure 12.
Figure 12 Completion of the review of various drug registration applications in 2015-2018
2. Completed category-specific reviews for registration application
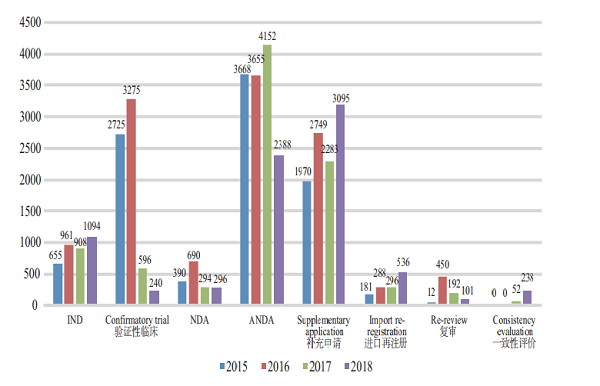
In 2018, CDE completed the reviews for 1,094 IND applications, 296 NDAs, and 2,388 ANDAs. The completion of the review of various drug registration applications in 2015-2018 is detailed in Figure 13.
Figure 13 Completion of the review of various drug registration applications in 2015-2018
3. Approved reviews
In 2018, CDE reviewed and approved (in the Annual Review Report of previous years, it was stated as passed the review and recommended for approval, the same below) 947 INDs, 175 NDAs, and 1,038 ANDAs.
CDE reviewed and approved the marketing of 9 varieties of Class 1 innovative drugs, and 67 varieties of imported brand-name drugs. For details, see Annexes 1 and 2.



