The 2018 Drug Review Annual Report released
3. Biological products
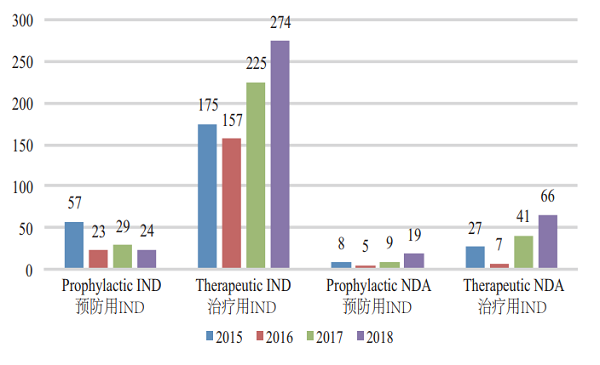
CDE accepted 944 applications for registration of biological products, covering 298 INDs; and 85 NDAs, up by 70% YOY. For such details, see Figure 8. The acceptance of registration applications for clinical trials and marketing of biologicals in 2015-2018 is detailed in Figure 9.
Figure 8 Acceptance of various registration applications for biologicals in 2018
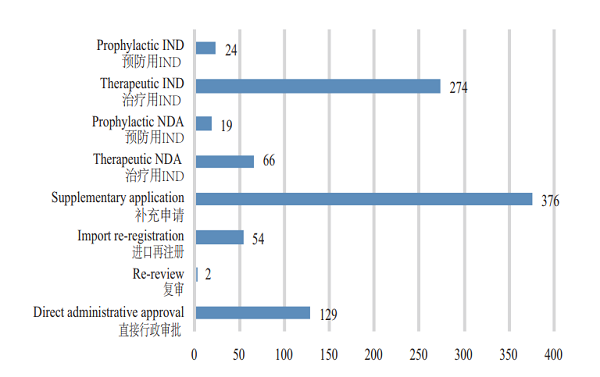
Figure 9 Acceptance of registration applications for clinical trials and marketing of biologicals in 2015-2018
(1) Class 1 innovative biologicals CDE accepted 123 applications for registration of Class 1 innovative biologicals (incl. 6 prophylactic biologicals and 117 therapeutic ones), an increase of 62% over 2017, covering 11 Class 1 NDAs (incl. 2 prophylactic products and 9 for therapy, involving 9 varieties), increasing 5.5 times than that of 2017; 112 Class 1 INDs (incl. 4 prophylactic products and 108 therapeutic ones, involving 97 varieties), an increase of 51% over 2017.
(2) Indications of INDs of Class 1 innovative biologicals for therapeutic use
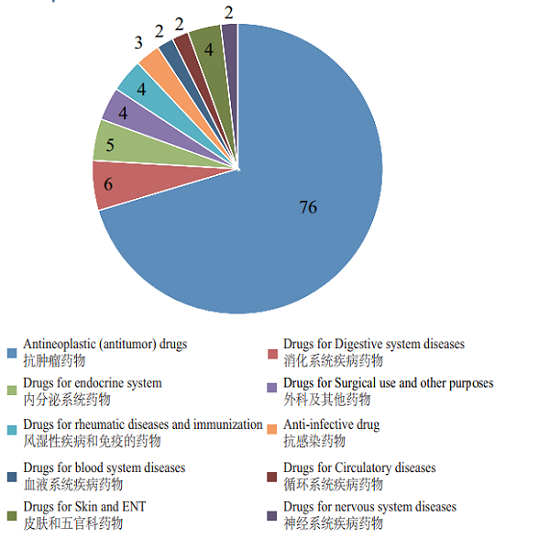
CDE accepted 108 IND applications for Class 1 therapeutic biological products (involving 93 varieties), 70% of which have indications mainly concentrated in the field of anti-tumor treatment, for specific therapeutic areas, see Figure 10.
Figure 10 Distribution of therapeutic areas of IND applications for Class 1 therapeutic biologicals accepted in 2018