The 2018 Drug Review Annual Report released
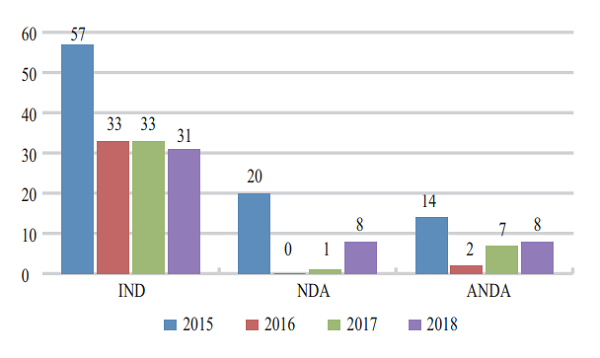
2. Acceptance of TCM registration applications CDE accepted 413 TCM registration applications, covering 31 applications for INDs, 8 NDAs, and 8 ANDAs. The details of the acceptance of registration applications for various TCMs are shown in Figure 6. The 2015-2018 acceptance of registration applications for TCM clinical trials and marketing is detailed in Figure 7.
Figure 6 Acceptance of various TCM registration applications in 2018
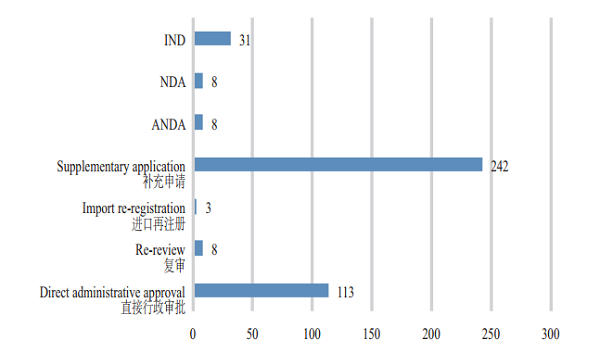
Figure 7 Acceptance of registration applications for TCM clinical trials and marketing in 2015-2018
(1) New TCMs
CDE accepted 39 applications for registration of Class 1-6 new TCMs, covering 8 NDAs (8 varieties involved), increasing 8 times than that in 2017; 31 INDs (incl. 29 varieties), 2 of which were for Class 1 innovative TCM (involving 1 variety).
(2) Indications of TCM INDs CDE accepted 31 applications for TCM INDs, 65% of which are with therapeutic areas mainly covering digestion, cardiovascular, respiratory and psychoneural system.



