The 2018 Drug Review Annual Report released
On July 1, 2019, the 2018 Drug Review Annual Report was released, which is excerpted as follows:
In 2018, under the strong leadership of the National Medical Products Administration (hereinafter referred to as NMPA), the Center for Drug Evaluation (hereinafter referred to as CDE) has, pursuant to the Opinions of the General Office of the CPC Central Committee and the General Office of the State Council on Deepening the Reform of Examination & Approval System to Encourage Innovation in Drugs and Medical Devices (General Office [2017] No. 42, hereinafter referred to as Document No. 42); the State Council's Opinions on Reforming the Review & Approval System for Drugs and Medical Devices (State Council [2015] No. 44 (hereinafter referred to as Document No. 44), and the requirements of the Standing Committee of the State Council on April 12 and June 20, performed a series of tasks to encourage innovation in drug R&D, improve drug quality, and guarantee drug safety, effectiveness and accessibility, etc. CDE has furthered the reform of the drug review & approval system with high responsibility and sense of mission, adhered to the law-based, scientific and standardized review, to resolutely safeguard and promote public health.
I. Acceptance of drug registration applications
In 2018, CDE accepted a total of 7,336 new registration applications (counted by acceptance numbers, the same below), of which 5,574 are subject to technical review, the rest to direct administrative examination and approval (dispense with technical review, the same below). Compared with 2017, the number of registration applications requiring technical review by CDE saw a substantial increase (up by 47%) in 2018, whereof the numbers of applications for registration of TCMs, chemicals and biologicals all saw a significant increase (up by 30%, 50% and 42%, respectively).
In 2018, CDE accepted the registration applications for a total of:
---264 varieties of innovative drugs (involving 533 acceptance numbers, the number of varieties of chemical drugs is based on the statistical analysis of active ingredients, while the varieties of TCM and biological products are all counted by their generic names), up by 21% YOY, covering 239 INDs (up by 15% YOY) and 25 NDAs (up by 150% YOY) for Class 1 innovative drugs.
--- 157 varieties of Class 1 chemicals, covering 16 NDAs (up by 100% YOY) for Class 1 innovative chemical drugs.
--- 37 varieties of Class 1-6 new TCMs, covering 8 NDAs (increased 8 times than that in 2017); and 29 INDs, one of which went for Class 1 TCM innovative drugs. --- 106 Class 1 innovative biologicals (an increase of 62% over 2017, involving a total of 123 acceptance numbers for 6 preventive biologicals and 117 therapeutic biologicals), covering 9 NDAs (involving 11 acceptance numbers, 2 for preventive biologicals and 9 for therapeutic biologicals), increased 5.5 times than that in 2017.
(I) Overall situation Of the 7,336 new registration applications accepted by CDE, those for chemical drugs accounted for 82% (5,979) of the total. For a four-year comparison, see Figure 1 for details.
Figure 1 Acceptance of various drug registration applications from 2015 to 2018
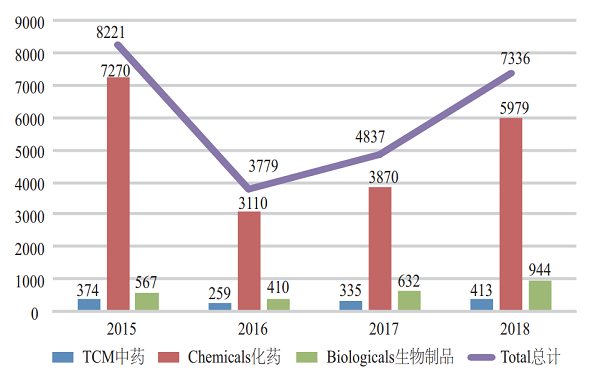
Among the 5,574 applications requiring technical review, 4,459 were for chemical drugs, accounting for 80% of all those for technical reviews, and 300 and 815 were for TCMs and biologicals, respectively.



