Home> Science Outreach
Researchers reveal novel mechanism for RNA m6A modification in regulating human stem cell senescence
Updated: 2020-10-13
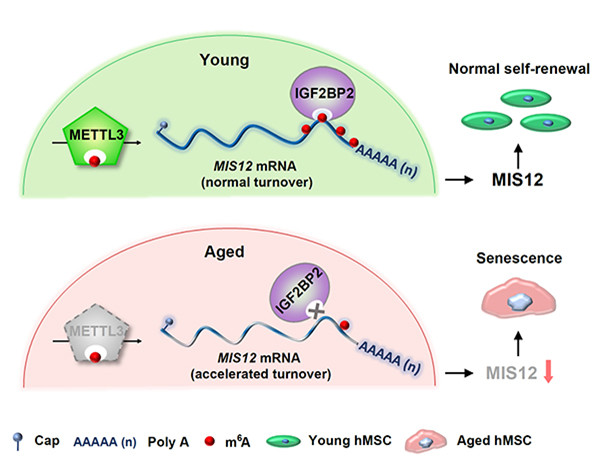
A diagram shows a model illustrating the protective role of METTL3/m6A in alleviating hMSC senescence through IGF2BP2-mediated enhancement of MIS12 mRNA stability. [Photo/WeChat account of Biophysical Society of China]
Research teams from the Institute of Zoology and Beijing Institute of Genomics at the Chinese Academy of Sciences collaborated and unveiled a novel mechanism of METTL3, as well as m6A in regulating human stem cell senescence, in a paper they published in the Nucleic Acids Research journal on Oct 9.
The paper -- entitled "METTL3 counteracts premature aging via m6A-dependent stabilization of MIS12 mRNA" -- described their research process and results in detail.
In the study, researchers initially found that the levels of both m6A RNA methylations and its METTL3 were reduced during human stem cell aging.
A knockout of METTL3 accelerated stem cell senescence, whereas overexpression of METTL3 rescued cellular senescence phenotypes. Transcriptional profiling of m6A modifications further identified MIS12, a cell cycle regulator, of which m6A modifications were reduced in both hMSCs and METTL3-deficient hMSCs.
Mechanistically, loss of m6A modifications decreased the expression and accelerated the turnover of MIS12 mRNA, while a knockout of MIS12 accelerated cellular senescence.
Furthermore, m6A reader IGF2BP2 was identified as a key player in recognizing and stabilizing m6A-modified MIS12 mRNA; a knockdown of IGF2BP2 also accelerated senescence in human stem cells.
Taken together, this study puts forth a regulatory model in which METTL3 and m6A modifications alleviate hMSC senescence through IGF2BP2-mediated enhancement of MIS12 mRNA stability.
The existence of a direct molecular connection between MIS12 expression and its m6A level suggests the manipulation of m6A modifications, or its core methyltransferase METTL3, as potential strategies for cellular rejuvenation.
This work sheds light on the poorly understood molecular mechanism of m6A in aging and identifies METLL3 and MIS12 as potential novel biomarkers and therapeutic targets for aging-associated disorders.
