Home> News
Chinese researchers make significant discovery in Parkinson's disease-related phosphorylation
Updated: 2020-08-11
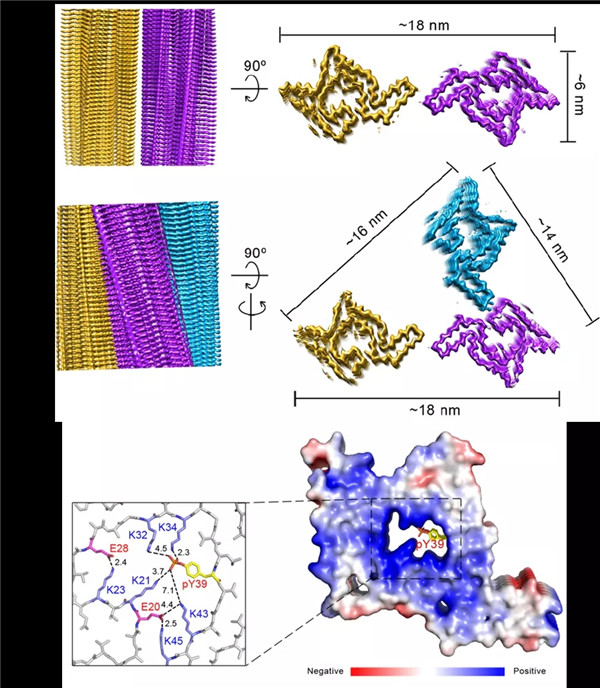
A diagram of the electron microscopic structure of pY39α-syn amyloid fibril. [Photo/WeChat account of BSC]
Chinese researchers have found that Parkinson's disease-related phosphorylation at Tyr39 rearranges α-synuclein amyloid fibril structure revealed by cryo-EM, according to news on the WeChat account of Biophysical Society of China (BSC).
A research team led by Li Cong from the Shanghai Institute of Organic Chemistry -- under the Chinese Academy of Sciences (CAS) -- and a research team led by Li Yanmei of Tsinghua University were involved. They have established a structural understanding of the pathology of the pY39 α-synuclein (α-syn) fibril and have highlighted the importance of posttranslational modifications (PTMs) in defining the polymorphism and pathology of amyloid fibrils in neurodegenerative diseases.
The PTMs of α-syn, e.g., phosphorylation, play an important role in modulating α-syn pathology in Parkinson's disease (PD) and α-synucleinopathies. Accumulation of phosphorylated α-syn fibrils in Lewy bodies and Lewy neurites is the histological hallmark of these diseases.
The study has been published in the Proceedings of the National Academy of Sciences of the United States of America (PNAS).
