Home>Updates
Lecheng conducts China's first application of innovative orthopedics instrument
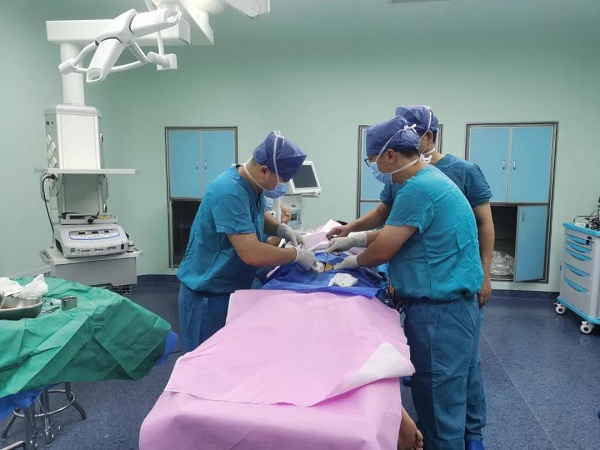
The surgery of the first application of the Zimmer Biomet GPS III platelet concentration system on the Chinese mainland.
The first application of a Zimmer Biomet GPS III platelet concentration system on the Chinese mainland was operated in the Lecheng branch of Hainan General Hospital in Hainan Boao Lecheng International Medical Tourism Pilot Zone on June 21.
The surgery was performed by Lin Jianping, director of the Orthopaedic Medical Center of Hainan General Hospital.
GPS III was originally produced by Zimmer Biomet, a global leader in musculoskeletal healthcare based in the United States. Currently, the product has been approved to enter the market in countries including the US and Japan for athletic injury treatment, especially for the acute injury of athletes.

The GPS III platelet concentration system.
The product's application in Lecheng was used to treat a track athlete who was diagnosed with tendon rupture, semitendinosus tendon rupture and semimembranosus injury before a competition.
The process for GPS III to gain approval for licensed operation in Lecheng took only one day thanks to the joint efforts of the hospital and other government departments such as the Health Commission of Hainan Province and Hainan Medical Products Administration.
"The application of GPS III is minimally invasive and can achieve a quick recovery. Compared with traditional treatment methods, this new product can relieve patient's pain remarkably in only a few days after the surgery and speed up the recovery of soft tissue and ligament injuries," said Lin.
The Boao Lecheng International Medical Tourism Pilot Zone Administration will further deepen its cooperation with Zimmer Biomet to start the licensed operation of more products to help patients in China with orthopedic diseases relieve their pain and improve their quality of life.

 Different approaches to COVID-19 vaccines
Different approaches to COVID-19 vaccines Boao Super Hospital
Boao Super Hospital Boao Yiling Life Care Center
Boao Yiling Life Care Center  Neology Stem Cell Anti-Aging Hospital
Neology Stem Cell Anti-Aging Hospital 